Composition and Properties
Polyethylene oxide (PEO), also known as polyethylene glycol (PEG), is an emulsifier (or thickening agent) used in many industrial, cosmetic, and food applications. PEOs are hydrophilic (attracted to water) and many fisters refer to PEO lubes as water-based lubes.
Lubricity is due to the incredible length PEO chains, with lubricant-grade PEOs having at least 90,000 units in a single molecule. In theory, PEOs should be good humectants; however, the physiological processes in the lower GI tract are stronger than the weak bonds between PEO and water. As soon as the body absorbs the water that was bonded to the PEO chain, both viscosity and lubricity decrease and drag increases. Rehydration or reapplication is necessary.
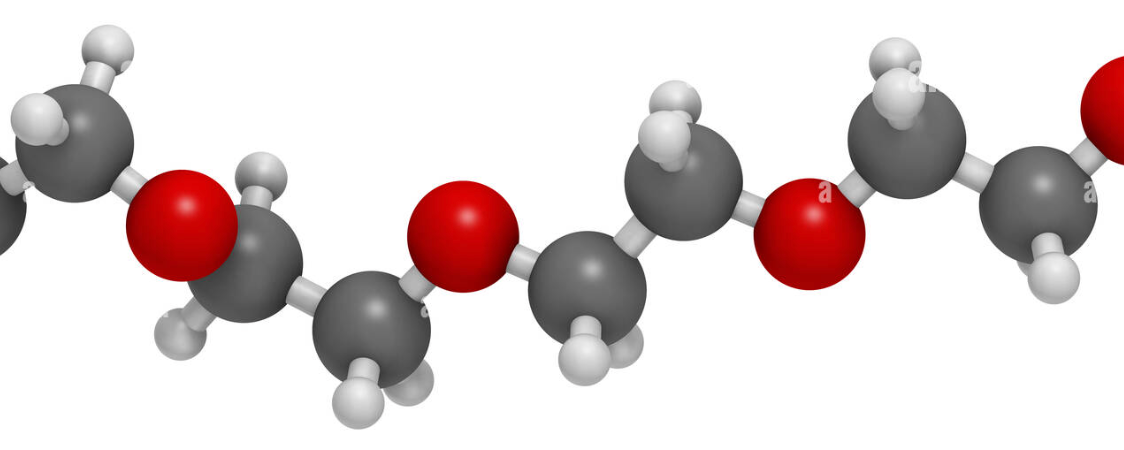
Figure 1.2: Structural Formula of PEO
The long chains of PEO lubricants include oxygen (red) connected to two carbons (dark gray). Each of these carbons are connected to hydrogen (light gray). When hydrated, dipole-dipole and weak hydrogen bonds form which are partially responsible for the unique characteristics of PEO lubricants.

/u_lubrication_(unambiguated)_lubrication_data_sheets.png)